Molecular Physiology

Chitin is one of the most abundant organic compounds on earth and becomes increasingly important as a renewable resource for the chemical/pharmaceutical industry. To obtain more insight into the process of chitin synthesis, we investigate the expression and regulation of the involved genes, analyze the functions of the derived proteins and dissect the reactions in which they participate. To allow comparative analyses and different experimental strategies, we address these questions in different insect model organisms (Aedes aegypti, Manduca sexta, Tribolium castaneum), which are amnenable to RNA interference allowing analysis of gene function. We use also the genetic model system Saccharomyces cerevisiae, because it allows us to analyze the intracellular maturation and trafficking of the proteins involved in chitin synthesis, and transfer the results to insect cells. The examined proteins including chitin synthases, chitin modifying enzymes and certain endopeptidases involved in molting are promising target sites for the action of novel environmentally safe insecticides and miticites. Actually, several classes of commercially used insecticides and miticides such as benzoylureas and diphenyloxazolines act as chitin synthesis inhibitors. Their mode of action, pharmacology, toxicology and resistance mechanisms are matters of particular interest to us and we address these questions using biochemical, genomic and proteomic tools. We also analyze the chitinous peritrophic matrix (PM), which acts as an infectious barrier in arthropod intestines, to understand the role of individual PM proteins and to identify antimicrobial proteins. We produce these proteins using bacterial and baculoviral expression systems and analyze their properties. Recombinant PM proteins are used to reconstitute chitinous hydrogels in vitro in order to generate new materials for biomedical applications. Other research projects in our group focus on chymotrypsin-like serine proeteases and ABC transporters involved in the elimination of insecticides and their metabolites contributing to insecticide resitance.
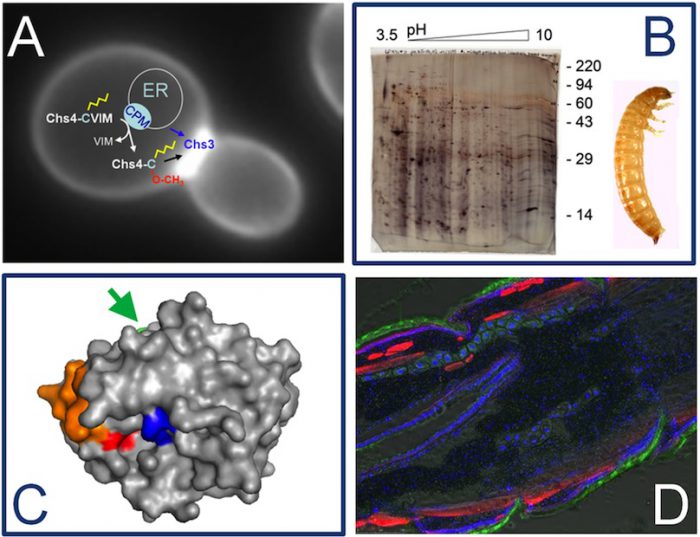
A) A budding yeast cell is shown in this image. The intracellular localization of the chitin synthase isoform Chs3 is visualized using a green fluorecent protein. Control of chitin synthase activity at the bud neck requires the regulatory subunit Chs4, which is prenylated at its C-terminal CaaX-motif. (B) 2D gel electrophoresis of proteins extracted from the larval midgut of Tribolium castaneum. This 2D gel was used to analyze the changes in the midgut proteome in response to treatement with a benzoylurea insecticide inhibiting chitin sythesis (C) Homology-based structural model of a chymotrypsin-like serine protease from the exuvial fluid of Tribolium castaneum. The catalytic site is depicted in blue, the specificity pocket in red, a surface loop in orange and a glycosylation site in green (arrow). We produce this and other enzymes as recombinat proteins in insect cells using a baculoviral expression system. This allows us to characterize substrate specificity and kinetic properties after site-specific mutagenesis. (D) Cryosection of a young Tribolium larva. Fixed sections were stained with different fluorescent dyes, to label nuclei (blue), actin filaments (red) and chitinous structures (green).


